Unraveling the Wonders of Proteins: Definitions, important points and examples
Introduction:
Proteins, the building blocks of life, are intricate molecules responsible for numerous vital functions in living organisms. Understanding their structure is crucial for unraveling their diverse roles in the human body and other organisms. In this blog post, we will delve into the world of protein structure, exploring key concepts such as amino acids, peptide bonds, and various levels of protein structure. Whether you are a student or a professional, this guide will equip you with essential knowledge to appreciate the complexity and beauty of proteins.
1. Amino Acid:
Definition:
Amino acids act as essential organic compounds, serving as the foundational components of proteins.
They consist of an amino group (-NH2) and a carboxyl group (-COOH) attached to a central carbon atom, along with a unique side chain (R group).
Important Points:
A diverse array of proteins arises from the combination of 20 standard amino acids in varying sequences.
Each amino acid's unique side chain imparts distinct chemical properties, influencing the protein's structure and function.
Amino acids are linked together through peptide bonds to form polypeptides, which then fold into functional protein structures.
Examples:
1. Glycine: The most basic amino acid consists of a hydrogen atom as its side chain.
2. Valine: Possesses a branched-chain structure, contributing to the hydrophobic nature of certain proteins.
3. Serine: Contains a hydroxyl group in its side chain, making it critical for phosphorylation and enzyme activity regulation.
2. Peptide Bond:
Definition:
Peptide bonds(covalent bond) form when the amino group of one amino acid bonds with the carboxyl group of another.
It results in the release of a water molecule (H2O) in a condensation reaction, linking the amino acids together to form a polypeptide chain.
Important Points:
The peptide bond's planar nature restricts rotation around its axis, influencing the polypeptide's folding and structure.
The sequence of amino acids in a polypeptide chain determines the protein's unique function and properties.
Proteins can range from short polypeptides with a few dozen amino acids to massive complexes with thousands of amino acids.
Examples:
1. In a dipeptide, two amino acids are linked by a single peptide bond.
2. A tripeptide consists of three amino acids connected by two peptide bonds.
3. An oligopeptide consists of a relatively small number of amino acids bonded together.
3. Polypeptide:
Definition:
A polypeptide forms as a chain of amino acids bond together via peptide bonds.
It serves as the precursor to a functional protein and can fold into specific three-dimensional shapes.
Important Points:
Polypeptides can be relatively short or exceedingly long, containing hundreds or thousands of amino acids.
The specific sequence of amino acids in a polypeptide dictates the protein's primary structure.
Multiple polypeptide chains may come together to form the complete protein structure.
Examples:
1. Insulin: A polypeptide hormone consisting of 51 amino acids, essential for regulating blood glucose levels.
2. Hemoglobin: Composed of four polypeptide chains (two alpha and two beta globins), responsible for oxygen transport in red blood cells.
3. Collagen: A fibrous protein made up of three polypeptide chains, providing structural support to connective tissues in the body.
4. Primary Structure:
Definition:
The primary structure of a protein refers to the linear sequence of amino acids in a polypeptide chain, connected by peptide bonds.
Important Points:
The primary structure is the foundation upon which the higher levels of protein structure are built.
A slight change in the amino acid sequence can lead to significant alterations in protein function and disease conditions.
The primary structure's genetic encoding is determined by the DNA sequence of the corresponding gene.
Examples:
1. Alpha-Synuclein: The primary structure of this protein is crucial in understanding its aggregation in Parkinson's disease.
2. Insulin Receptor: Genetic mutations in this receptor's primary structure can result in insulin resistance and diabetes.
3. p53 Tumor Suppressor: A critical protein in cell cycle regulation, mutations in its primary structure can lead to cancer.
5. Secondary Structure:
Definition:
Secondary structure of a protein denotes the localized folding patterns formed by hydrogen bonding between the polypeptide backbone constituents, including alpha-helices and beta-sheets.
Important Points:
The secondary structure stabilizes the protein through interactions between backbone atoms, leading to unique geometric arrangements.
Hydrogen bonds between carbonyl and amino groups of nearby amino acids contribute to the formation of secondary structures.
Secondary structures can occur independently or in combination within a single protein molecule.
Examples:
1. Alpha-Helix: Found in proteins such as keratin and myoglobin, the alpha-helix resembles a spiral staircase.
2. Beta-Sheet: Present in proteins like silk and fibroin, beta-sheets consist of extended strands linked by hydrogen bonds.
3. Coiled-Coil: A specialized secondary structure formed by two or more alpha-helices winding around each other, as seen in proteins like myosin.
6. Tertiary Structure:
Definition:
Tertiary structure of a protein represents its three-dimensional conformation arrangement, resulting from interactions between amino acid side chains and the polypeptide backbone.
Important Points:
Tertiary structure plays a crucial role in determining a protein's function, stability, and specificity.
Forces influencing tertiary structure include hydrophobic interactions, hydrogen bonds, disulfide bridges, and van der Waals forces.
The overall folding pattern may result in a globular or fibrous protein shape.
Examples:
1. Hemoglobin: The unique tertiary structure of hemoglobin facilitates oxygen binding and release in red blood cells.
2. Ribonuclease A: The precise folding of this enzyme enables it to cleave RNA molecules.
3. Immunoglobulins (Antibodies): Tertiary structure diversity in antibodies allows them to recognize and bind specific antigens.
7. Quaternary Structure:
Definition:
The quaternary structure of a protein refers to the arrangement of multiple polypeptide chains (subunits) in a functional protein complex.
Important Points:
Proteins with quaternary structure exhibit a higher level of organization, composed of two or more individual polypeptides.
The arrangement of subunits is crucial for the protein's overall function and regulation.
Some proteins exist as functional complexes only in their quaternary form.
Examples:
1. Hemoglobin: Exhibits quaternary structure as a tetramer, with four globin chains (subunits) coming together.
2. DNA Polymerase: A multi-subunit protein complex responsible for DNA replication in cells.
3. Muscle Myosin: Composed of multiple polypeptide chains, the quaternary structure allows for muscle contraction.
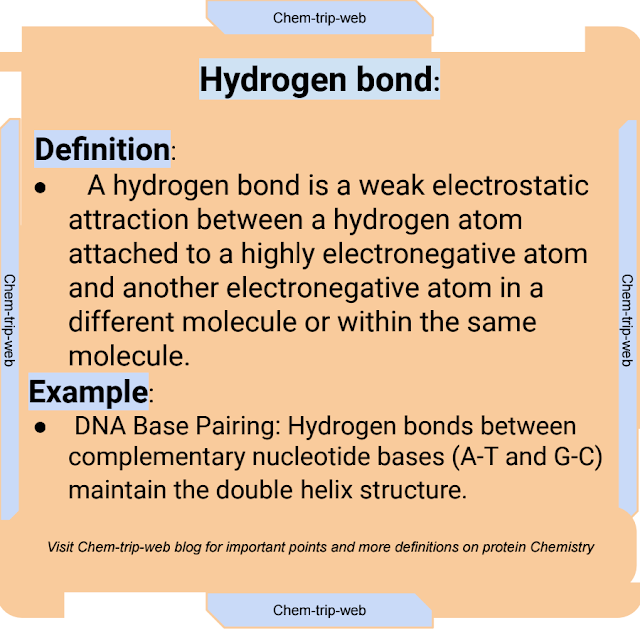
8. Hydrogen Bond:
Definition:
A hydrogen bond is a weak electrostatic attraction between a hydrogen atom attached to a highly electronegative atom and another electronegative atom in a different molecule or within the same molecule.
Important Points:
Hydrogen bonds play a vital role in stabilizing protein secondary and tertiary structures.
The most common hydrogen bonds in proteins form between the amide nitrogen and carbonyl oxygen of the polypeptide backbone.
Hydrogen bonds are crucial in DNA base pairing and maintaining the three-dimensional structure of water molecules.
Examples:
1. Alpha-Helix:
Hydrogen bonds between the carbonyl oxygen and amide hydrogen atoms stabilize the helical structure.
2. DNA Base Pairing: Hydrogen bonds between complementary nucleotide bases (A-T and G-C) maintain the double helix structure.
3. Enzyme-Substrate Interaction: Hydrogen bonds facilitate specific interactions between enzyme active sites and substrate molecules.
9. Disulfide Bond:
Definition:
A disulfide bond is a covalent bond formed between two cysteine amino acid residues in a protein, resulting from the oxidation of thiol groups (-SH) on their side chains.
Important Points:
Disulfide bonds contribute to the tertiary and quaternary structure stabilization in proteins.
These bonds are essential for maintaining protein conformation and stability in extracellular environments.
Disulfide bonds play a role in protein folding and disulfide isomerization during post-translational modifications.
Examples:
1. Insulin: Disulfide bonds stabilize the insulin protein, ensuring proper folding and biological activity.
2. Immunoglobulins (Antibodies): Disulfide bonds help form the Y-shaped structure of antibodies, enabling antigen recognition.
3. Keratin: The formation of disulfide bonds is crucial for the structural integrity of hair and nails.
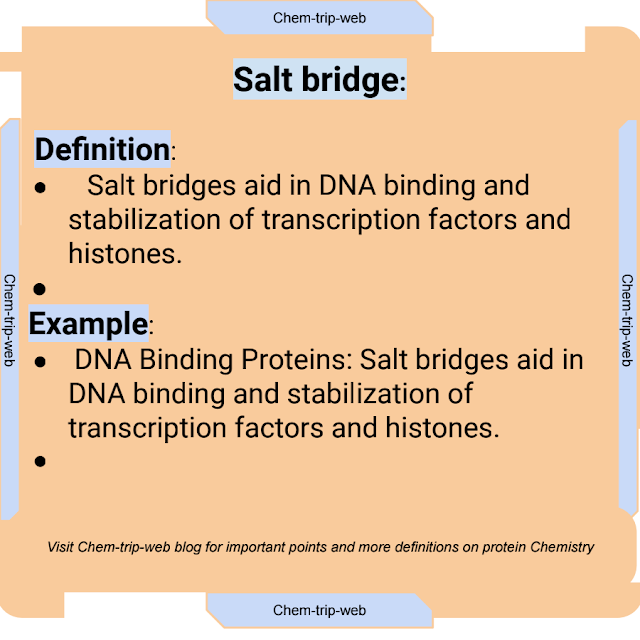
10. Salt Bridge:
Definition:
A salt bridge, also known as ionic bridge or ionic interaction, is an electrostatic attraction between positively charged and negatively charged amino acid side chains in a protein.
Important Points:
Salt bridges contribute to the stabilization of protein tertiary and quaternary structures.
These ionic interactions can occur within a single polypeptide chain or between different polypeptide chains
Salt bridges play a role in protein-protein interactions and protein-ligand binding.
Examples:
1. Hemoglobin: Salt bridges between oppositely charged amino acids in different globin subunits contribute to the protein's quaternary structure.
2. Enzyme-Substrate Interaction: Salt bridges between charged amino acids in the enzyme active site and substrate facilitate enzyme catalysis.
3. DNA Binding Proteins: Salt bridges aid in DNA binding and stabilization of transcription factors and histones.
( Post is written, edited, proofread, grammar quality and content checked using AI tools)




Comments
Post a Comment